|
When
different kinds of oil enter the sea, many physical, chemical
and biological degradation processes start acting on them. These
processes change the properties and behaviour of the oil. Some
processes cause the oil to "disappear", but the fact
that it is no longer visible on the water surface does not necessarily
mean that it is gone or has been rendered environmentally harmless.
Every
time oil enters the sea, a number of factors will decide the
physical, chemical and biological degradation of the oil,
as well as the potential environmental damage (see effects
on wildlife/species and effects
on habitats) of the spill or discharge, in that particular
area:
- The
composition and amount of oil discharged (see, e.g., NOAA).
- The
quantity and duration of the discharge/spill.
- The
time of year at which it occurs.
- The
temperature of the air and the receiving water body.
- The
weather conditions.
- The
species composition in the area affected.
- The
properties of the shore line (rocky, sandy, mud flats, mangroves,
etc.)
- The
amount of oil-degrading micro-organisms in the area.
- The
supply of oxygen in the water
- The
following description (green text)
of physical, chemical and biological degradation processes
can be found on the site of the International Tanker Owners
Pollution Federation Ltd (ITOPF).
- Red
text
= complementing text from similar descriptions by other
organizations/agencies (see below: EPA, AMSA, NOAA, Offshore
environment). See also NOAA photos
of different types and distributions of oil on water.
Fate
of oil spilled, showing the main weathering processes. Graph
courtesy of ITOP. © ITOPF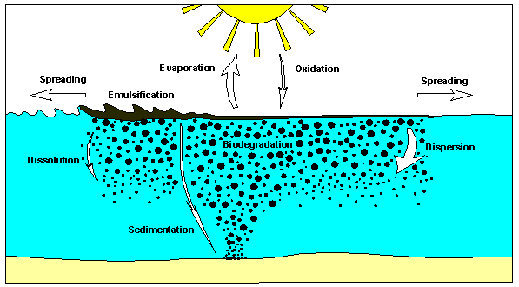
Weathering,
sheen, windrows, spreading, dispersion, evaporation, dissolution,
biodegradation, sedimentation, emulsification, dissolution,
oxidation, aggregation ...
 "Oil,
when spilled at sea, will normally break up and be dissipated
or scattered into the marine environment over time. This dissipation
is a result of a number of chemical and physical processes
that change the compounds that make up oil when it is spilled.
The processes are collectively known as weathering.
Oils weather in different ways. Some of the processes, like
natural dispersion of the oil into the water, cause part of
the oil to leave the sea surface, while others, like evaporation
or the formation of water in oil emulsions, cause the oil
that remains on the surface to become more persistent.
"Oil,
when spilled at sea, will normally break up and be dissipated
or scattered into the marine environment over time. This dissipation
is a result of a number of chemical and physical processes
that change the compounds that make up oil when it is spilled.
The processes are collectively known as weathering.
Oils weather in different ways. Some of the processes, like
natural dispersion of the oil into the water, cause part of
the oil to leave the sea surface, while others, like evaporation
or the formation of water in oil emulsions, cause the oil
that remains on the surface to become more persistent.
 Weathering:
Winds, waves and currents may result in natural dispersion,
breaking a slick into droplets which re then distributed throughout
the water. These droplets may also result in the creation
of a secondary slick or thin film (sheen) on the surface of
the water. (EPA) •••
A sheen is a very thin layer (less than 0.003 mm thick)
floating on the water surface, and the most commonly-observed
form of oil during the later stages of a spill. Depending
on thickness, sheens range in colour from dull brown for the
thickest ones to rainbows, gray, silvers, and near-transparency
in the case of the thinnest sheens. Windrows are streaks
of oil that line up in the direction of the wind (typically
early during a spill, when the wind speed is at least 5 metres
per second). Sheen is the form of oil that most frequently
windrows. (NOAA)
Weathering:
Winds, waves and currents may result in natural dispersion,
breaking a slick into droplets which re then distributed throughout
the water. These droplets may also result in the creation
of a secondary slick or thin film (sheen) on the surface of
the water. (EPA) •••
A sheen is a very thin layer (less than 0.003 mm thick)
floating on the water surface, and the most commonly-observed
form of oil during the later stages of a spill. Depending
on thickness, sheens range in colour from dull brown for the
thickest ones to rainbows, gray, silvers, and near-transparency
in the case of the thinnest sheens. Windrows are streaks
of oil that line up in the direction of the wind (typically
early during a spill, when the wind speed is at least 5 metres
per second). Sheen is the form of oil that most frequently
windrows. (NOAA)
 The
way in which an oil slick breaks up and dissipates depends
largely on how persistent the oil is. Light products such
as kerosene tend to evaporate and dissipate quickly and naturally
and rarely need cleaning-up. These are called non-persistent
oils. In contrast, persistent oils, such as many crude oils,
break up and dissipate more slowly and usually require a clean-up
response. Physical properties such as the density, viscosity
and pour point of the oil all affect its behaviour.
The
way in which an oil slick breaks up and dissipates depends
largely on how persistent the oil is. Light products such
as kerosene tend to evaporate and dissipate quickly and naturally
and rarely need cleaning-up. These are called non-persistent
oils. In contrast, persistent oils, such as many crude oils,
break up and dissipate more slowly and usually require a clean-up
response. Physical properties such as the density, viscosity
and pour point of the oil all affect its behaviour.
 Dissipation
does not occur immediately. The time this takes depends on
a series of factors, including the amount and type of oil
spilled, the weather conditions and whether the oil stays
at sea or is washed ashore. Sometimes, the process is quick
and on other occasions it can be slow, especially in sheltered
and calm areas of water.
Dissipation
does not occur immediately. The time this takes depends on
a series of factors, including the amount and type of oil
spilled, the weather conditions and whether the oil stays
at sea or is washed ashore. Sometimes, the process is quick
and on other occasions it can be slow, especially in sheltered
and calm areas of water.
 Spreading:
As soon as oil is spilled,
it starts to spread out over the sea surface, initially as
a single slick. The speed at which this takes place depends
to a great extent upon the viscosity of the oil. Fluid, low
viscosity oils spread more quickly than those with a high
viscosity. Nevertheless, slicks quickly spread to cover extensive
areas of the sea surface. Spreading is rarely uniform and
large variations in the thickness of the oil are typical.
After a few hours the slick will begin to break up and, because
of winds, wave action and water turbulence, will then form
narrow bands or windrows parallel to the wind direction. The
rate at which the oil spreads is also determined by the prevailing
conditions such as temperature, water currents, tidal streams
and wind speeds. The more severe the conditions, the more
rapid the spreading and breaking up of the oil.
Spreading:
As soon as oil is spilled,
it starts to spread out over the sea surface, initially as
a single slick. The speed at which this takes place depends
to a great extent upon the viscosity of the oil. Fluid, low
viscosity oils spread more quickly than those with a high
viscosity. Nevertheless, slicks quickly spread to cover extensive
areas of the sea surface. Spreading is rarely uniform and
large variations in the thickness of the oil are typical.
After a few hours the slick will begin to break up and, because
of winds, wave action and water turbulence, will then form
narrow bands or windrows parallel to the wind direction. The
rate at which the oil spreads is also determined by the prevailing
conditions such as temperature, water currents, tidal streams
and wind speeds. The more severe the conditions, the more
rapid the spreading and breaking up of the oil.
 Physical
transport. Only ten minutes after a spill of 1 ton of oil,
the oil can disperse over a radius of 50 metres, forming a
slick 10 millimetres thick. The slick gets thinner (less than
one mm) as oil continues to spread, covering an area of up
to 12 sq.km. --- Further changes take place under the combined
impact of meteorological and hydrological factors, and depend
mainly on the power and direction of wind, waves and currents.
An oil slick usually drifts in the same direction as the wind.
While the slick thins, especially after the critical thickness
of about 0.1 mm, it disintegrates into separate fragments
that spread over larger and more distant areas. Storms and
active turbulence speed up the dispersion of the slick
and its fragments. A considerable part of the oil disperses
in the water as fine droplets that can be transported over
large distances away from the place of the spill. (Offshore
Environment)
Physical
transport. Only ten minutes after a spill of 1 ton of oil,
the oil can disperse over a radius of 50 metres, forming a
slick 10 millimetres thick. The slick gets thinner (less than
one mm) as oil continues to spread, covering an area of up
to 12 sq.km. --- Further changes take place under the combined
impact of meteorological and hydrological factors, and depend
mainly on the power and direction of wind, waves and currents.
An oil slick usually drifts in the same direction as the wind.
While the slick thins, especially after the critical thickness
of about 0.1 mm, it disintegrates into separate fragments
that spread over larger and more distant areas. Storms and
active turbulence speed up the dispersion of the slick
and its fragments. A considerable part of the oil disperses
in the water as fine droplets that can be transported over
large distances away from the place of the spill. (Offshore
Environment)
 Evaporation: Lighter
components of the oil will evaporate to the atmosphere. The
amount of evaporation and the speed at which it occurs depend
upon the volatility of the oil. An oil with a large percentage
of light and volatile compounds will evaporate more than one
with a larger amount of heavier compounds. For example, petrol,
kerosene and diesel oils, all light products, tend to evaporate
almost completely in a few days whilst little evaporation will
occur from a heavy fuel oil. In general, in temperate conditions,
those components of the oil with a boiling point under 200ºC
tend to evaporate within the first 24 hours. Evaporation can
increase as the oil spreads, due to the increased surface area
of the slick. Rougher seas, high wind speeds and high temperatures
also tend to increase the rate of evaporation and the proportion
of an oil lost by this process.
Evaporation: Lighter
components of the oil will evaporate to the atmosphere. The
amount of evaporation and the speed at which it occurs depend
upon the volatility of the oil. An oil with a large percentage
of light and volatile compounds will evaporate more than one
with a larger amount of heavier compounds. For example, petrol,
kerosene and diesel oils, all light products, tend to evaporate
almost completely in a few days whilst little evaporation will
occur from a heavy fuel oil. In general, in temperate conditions,
those components of the oil with a boiling point under 200ºC
tend to evaporate within the first 24 hours. Evaporation can
increase as the oil spreads, due to the increased surface area
of the slick. Rougher seas, high wind speeds and high temperatures
also tend to increase the rate of evaporation and the proportion
of an oil lost by this process.
 Evaporation
occurs
when the lighter substances within the oil mixture become
vapors and leave the surface of the water. This process leaves
behind the heavier components of the oil, which may undergo
further weathering or may sink to the ocean floor. For example,
spills of lighter refined petroleum-based products such as
kerosene and gasoline contain a high proportion of flammable
components known as light ends. These may evaporate completely
within a few hours, thereby reducing the toxic effects to
the environment. Heavier oils leave a thicker, more viscous
residue, which may have serious physical and chemical impacts
on the environment. Wind, waves, and currents increase both
evaporation and natural dispersion. (EPA)
Evaporation
occurs
when the lighter substances within the oil mixture become
vapors and leave the surface of the water. This process leaves
behind the heavier components of the oil, which may undergo
further weathering or may sink to the ocean floor. For example,
spills of lighter refined petroleum-based products such as
kerosene and gasoline contain a high proportion of flammable
components known as light ends. These may evaporate completely
within a few hours, thereby reducing the toxic effects to
the environment. Heavier oils leave a thicker, more viscous
residue, which may have serious physical and chemical impacts
on the environment. Wind, waves, and currents increase both
evaporation and natural dispersion. (EPA)
 Dispersion: Waves
and turbulence at the sea surface can cause all or part of
a slick to break up into fragments and droplets of varying
sizes. These become mixed into the upper levels of the water
column. Some of the smaller droplets will remain suspended
in the sea water while the larger ones will tend to rise back
to the surface, where they may either coalesce with other
droplets to reform a slick or spread out to form a very thin
film. The oil that remains suspended in the water has a greater
surface area than before dispersion occurred. This encourages
other natural processes such as dissolution, biodegradation
and sedimentation to occur. The speed at which an oil
disperses is largely dependent upon the nature of the oil
and the sea state, and occurs most quickly if the oil is light
and of low viscosity and if the sea is very rough. These factors
led to the complete dispersion of the oil spilled from the
Braer (Shetland Islands) in 1993. The addition of chemical
dispersants (also surfactants) can accelerate this
process of natural dispersion.
Dispersion: Waves
and turbulence at the sea surface can cause all or part of
a slick to break up into fragments and droplets of varying
sizes. These become mixed into the upper levels of the water
column. Some of the smaller droplets will remain suspended
in the sea water while the larger ones will tend to rise back
to the surface, where they may either coalesce with other
droplets to reform a slick or spread out to form a very thin
film. The oil that remains suspended in the water has a greater
surface area than before dispersion occurred. This encourages
other natural processes such as dissolution, biodegradation
and sedimentation to occur. The speed at which an oil
disperses is largely dependent upon the nature of the oil
and the sea state, and occurs most quickly if the oil is light
and of low viscosity and if the sea is very rough. These factors
led to the complete dispersion of the oil spilled from the
Braer (Shetland Islands) in 1993. The addition of chemical
dispersants (also surfactants) can accelerate this
process of natural dispersion.
 Emulsification:
An emulsion is formed when two liquids combine, with one ending
up suspended in the other. Emulsification of crude oils refers
to the process whereby sea water droplets become suspended
in the oil. This occurs by physical mixing promoted by turbulence
at the sea surface. The emulsion thus formed is usually very
viscous and more persistent than the original oil and is often
referred to as chocolate mousse because of its appearance.
The formation of these emulsions causes the volume of pollutant
to increase between three and four times. This slows and delays
other processes which would allow the oil to dissipate. Oils
with an asphaltene content greater than 0.5% tend to form
stable emulsions which may persist for many months after the
initial spill has occurred. Those oils containing a lower
percentage of asphaltenes are less likely to form emulsions
and are more likely to disperse. Emulsions may separate into
oil and water again if heated by sunlight under calm conditions
or when stranded on shorelines.
Emulsification:
An emulsion is formed when two liquids combine, with one ending
up suspended in the other. Emulsification of crude oils refers
to the process whereby sea water droplets become suspended
in the oil. This occurs by physical mixing promoted by turbulence
at the sea surface. The emulsion thus formed is usually very
viscous and more persistent than the original oil and is often
referred to as chocolate mousse because of its appearance.
The formation of these emulsions causes the volume of pollutant
to increase between three and four times. This slows and delays
other processes which would allow the oil to dissipate. Oils
with an asphaltene content greater than 0.5% tend to form
stable emulsions which may persist for many months after the
initial spill has occurred. Those oils containing a lower
percentage of asphaltenes are less likely to form emulsions
and are more likely to disperse. Emulsions may separate into
oil and water again if heated by sunlight under calm conditions
or when stranded on shorelines.
 Emulsification
is a process that forms emulsions consisting of a mixture
of small droplets of oil and water. Emulsions are formed by
wave action, and greatly hamper weathering and cleanup processes.
Two types of emulsions exist: water-in-oil and oil-in-water.
Water-in-oil emulsions are frequently called "chocolate
mousse", and they are formed when strong currents
or wave action causes water to become trapped inside viscous
oil. Chocolate mousse emulsions may linger in the environment
for months or even years. Oil and water emulsions cause oil
to sink and disappear from the surface, which give the false
impression that it is gone and the threat to the environment
has ended. (EPA) •••
Milk is an example of an oil-in-water emulsion; butter on
the other hand is a water-in-oil emulsion. Both types of emulsification
require wave action and occur only for specific oil compositions.
Water-in-oil emulsions are extremely stable and may persist
for months or years after a spill. Water-in-oil emulsions
containing 50 to 80 per cent water are most common, and have
a reddish-brown colour and grease like consistency. They are
some times called "chocolate mousse" because
of their pudding-like appearance. (AMSA)
Emulsification
is a process that forms emulsions consisting of a mixture
of small droplets of oil and water. Emulsions are formed by
wave action, and greatly hamper weathering and cleanup processes.
Two types of emulsions exist: water-in-oil and oil-in-water.
Water-in-oil emulsions are frequently called "chocolate
mousse", and they are formed when strong currents
or wave action causes water to become trapped inside viscous
oil. Chocolate mousse emulsions may linger in the environment
for months or even years. Oil and water emulsions cause oil
to sink and disappear from the surface, which give the false
impression that it is gone and the threat to the environment
has ended. (EPA) •••
Milk is an example of an oil-in-water emulsion; butter on
the other hand is a water-in-oil emulsion. Both types of emulsification
require wave action and occur only for specific oil compositions.
Water-in-oil emulsions are extremely stable and may persist
for months or years after a spill. Water-in-oil emulsions
containing 50 to 80 per cent water are most common, and have
a reddish-brown colour and grease like consistency. They are
some times called "chocolate mousse" because
of their pudding-like appearance. (AMSA)
 Dissolution: Water
soluble compounds in an oil may dissolve into the surrounding
water. This depends on the composition and state of the oil,
and occurs most quickly when the oil is finely dispersed in
the water column. Components that are most soluble in sea
water are the light aromatic hydrocarbons compounds, such
as benzene and toluene. However, these compounds are also
those first to be lost through evaporation, a process
which is 10-100 times faster than dissolution. Oil contains
only small amounts of these compounds making dissolution one
of the less important processes.
Dissolution: Water
soluble compounds in an oil may dissolve into the surrounding
water. This depends on the composition and state of the oil,
and occurs most quickly when the oil is finely dispersed in
the water column. Components that are most soluble in sea
water are the light aromatic hydrocarbons compounds, such
as benzene and toluene. However, these compounds are also
those first to be lost through evaporation, a process
which is 10-100 times faster than dissolution. Oil contains
only small amounts of these compounds making dissolution one
of the less important processes.
 Oxidation: Oils
react chemically with oxygen either breaking down into soluble
products or forming persistent compounds called tars. This
process is promoted by sunlight and the extent to which it
occurs depends on the type of oil and the form in which it
is exposed to sunlight. However, this process is very slow
and even in strong sunlight, thin films of oil break down
at no more than 0.1% per day. The formation of tars
is caused by the oxidation of thick layers of high viscosity
oils or emulsions. This process forms an outer protective
coating of heavy compounds that results in the increased persistence
of the oil as a whole. Tar balls, which are often found
on shorelines and have a solid outer crust surrounding a softer,
less weathered interior, are a typical example of this process.
Oxidation: Oils
react chemically with oxygen either breaking down into soluble
products or forming persistent compounds called tars. This
process is promoted by sunlight and the extent to which it
occurs depends on the type of oil and the form in which it
is exposed to sunlight. However, this process is very slow
and even in strong sunlight, thin films of oil break down
at no more than 0.1% per day. The formation of tars
is caused by the oxidation of thick layers of high viscosity
oils or emulsions. This process forms an outer protective
coating of heavy compounds that results in the increased persistence
of the oil as a whole. Tar balls, which are often found
on shorelines and have a solid outer crust surrounding a softer,
less weathered interior, are a typical example of this process.
 Oxidation
occurs when oil contacts the water and oxygen combines with
the oil to produce water-soluble compounds. This process affects
oil slicks mostly around their edges. Thick slicks may only
partially oxidize, forming tar balls. These dense, sticky,
black spheres may linger in the environment, and can collect
in the sediments of slow moving streams or lakes or wash up
on shorelines long after a spill. (EPA)
••• Aggregation.
Oil aggregates in the form of petroleum lumps, tar balls or
pelagic tar, can be found both in the open and coastal waters
and on beaches. They derive from crude oil after the evaporation
and dissolution of its relatively light fractions, emulsification
of oil residuals, and chemical and microbial transformation.
- - - Oil aggregates look like light gray, brown, dark brown,
or black sticky lumps. They have an even shape and vary from
1 mm to 10 cm in size, sometimes reaching up to 50 cm. Their
surface serves as a substrate for developing bacteria, unicellular
algae and other microorganisms. Besides, many invertebrates,
like gastropods, polychaetes and crustaceans, resistant to
oil impact often use them as shelter. Oil aggregates can exist
from a month to a year in enclosed seas and up to several
years in the open ocean. They complete their cycle by slowly
degrading in the water column, on the shore if washed their
by currents, or (if they lose their floating ability) on the
sea bottom. (Offshore Environment)
Oxidation
occurs when oil contacts the water and oxygen combines with
the oil to produce water-soluble compounds. This process affects
oil slicks mostly around their edges. Thick slicks may only
partially oxidize, forming tar balls. These dense, sticky,
black spheres may linger in the environment, and can collect
in the sediments of slow moving streams or lakes or wash up
on shorelines long after a spill. (EPA)
••• Aggregation.
Oil aggregates in the form of petroleum lumps, tar balls or
pelagic tar, can be found both in the open and coastal waters
and on beaches. They derive from crude oil after the evaporation
and dissolution of its relatively light fractions, emulsification
of oil residuals, and chemical and microbial transformation.
- - - Oil aggregates look like light gray, brown, dark brown,
or black sticky lumps. They have an even shape and vary from
1 mm to 10 cm in size, sometimes reaching up to 50 cm. Their
surface serves as a substrate for developing bacteria, unicellular
algae and other microorganisms. Besides, many invertebrates,
like gastropods, polychaetes and crustaceans, resistant to
oil impact often use them as shelter. Oil aggregates can exist
from a month to a year in enclosed seas and up to several
years in the open ocean. They complete their cycle by slowly
degrading in the water column, on the shore if washed their
by currents, or (if they lose their floating ability) on the
sea bottom. (Offshore Environment)
 Sedimentation/Sinking: Some
heavy refined products have densities greater than one and
so will sink in fresh or brackish water. However sea water
has a density of approximately 1.025 and very few crudes are
dense enough or weather sufficiently, so that their residues
will sink in the marine environment. Sinking usually occurs
due to the adhesion of particles of sediment or organic matter
to the oil. Shallow waters are often laden with suspended
solids providing favourable conditions for sedimentation.
Oil stranded on sandy shorelines often becomes mixed with
sand and other sediments. If this mixture is subsequently
washed off the beach back into the sea it may then sink. In
addition, if the oil catches fire after it has been spilled,
the residues that sometimes form can be sufficiently dense
to sink.
Sedimentation/Sinking: Some
heavy refined products have densities greater than one and
so will sink in fresh or brackish water. However sea water
has a density of approximately 1.025 and very few crudes are
dense enough or weather sufficiently, so that their residues
will sink in the marine environment. Sinking usually occurs
due to the adhesion of particles of sediment or organic matter
to the oil. Shallow waters are often laden with suspended
solids providing favourable conditions for sedimentation.
Oil stranded on sandy shorelines often becomes mixed with
sand and other sediments. If this mixture is subsequently
washed off the beach back into the sea it may then sink. In
addition, if the oil catches fire after it has been spilled,
the residues that sometimes form can be sufficiently dense
to sink.
 Biodegradation:
Sea water contains a range of micro-organisms or microbes that
can partially or completely degrade oil to water soluble compounds
and eventually to carbon dioxide and water. Many types of microbe
exist and each tends to degrade a particular group of compounds
in crude oil. However, some compounds in oil are very resistant
to attack and may not degrade.
Biodegradation:
Sea water contains a range of micro-organisms or microbes that
can partially or completely degrade oil to water soluble compounds
and eventually to carbon dioxide and water. Many types of microbe
exist and each tends to degrade a particular group of compounds
in crude oil. However, some compounds in oil are very resistant
to attack and may not degrade.
The main factors affecting the efficiency of biodegradation,
are the levels of nutrients (nitrogen and phosphorus) in the
water, the temperature and the level of oxygen present. As biodegradation
requires oxygen, this process can only take place at the oil-water
interface since no oxygen is available within the oil itself.
The creation of oil droplets, either by natural or chemical
dispersion, increases the surface area of the oil and increases
the area available for biodegradation to take place."
 Many
species of marine micro-organisms or bacteria, fungi and yeasts
feed on the compounds that make up oil. Hydrocarbons (oil)
consumed by these micro-organisms can be partially metabolized
or completely metabolized to carbon dioxide and water. The
rate of biodegradation depends on the temperature of the oil
and water mixture. (AMSA). ••• A wide range
of micro-organisms is required for a significant reduction
of the oil. To sustain biodegradation, nutrients such as nitrogen
and phosphorus are sometimes added to the water to encourage
the micro-organisms to grow and reproduce. Biodegradation
tends to work best in warm water environments. (EPA) •••
The addition of nutrients to speed up the process is also
referred to as bioaugmentation, biostimulation, bioremediation,
seeding, or fertilization.
Many
species of marine micro-organisms or bacteria, fungi and yeasts
feed on the compounds that make up oil. Hydrocarbons (oil)
consumed by these micro-organisms can be partially metabolized
or completely metabolized to carbon dioxide and water. The
rate of biodegradation depends on the temperature of the oil
and water mixture. (AMSA). ••• A wide range
of micro-organisms is required for a significant reduction
of the oil. To sustain biodegradation, nutrients such as nitrogen
and phosphorus are sometimes added to the water to encourage
the micro-organisms to grow and reproduce. Biodegradation
tends to work best in warm water environments. (EPA) •••
The addition of nutrients to speed up the process is also
referred to as bioaugmentation, biostimulation, bioremediation,
seeding, or fertilization.
|



